Rotterdam, 18 June 2024
The Economist wrote in a tweet in December 2023 “Hydrogen is spectacularly useful. It could be used to power vehicles and generate electricity. It also emits no greenhouse gases, so it could help save the planet. But there is a downside: it is costly to produce.”.
Let’s start by elaborating on this quote, regarding why hydrogen is so spectacularly useful and costly to produce.
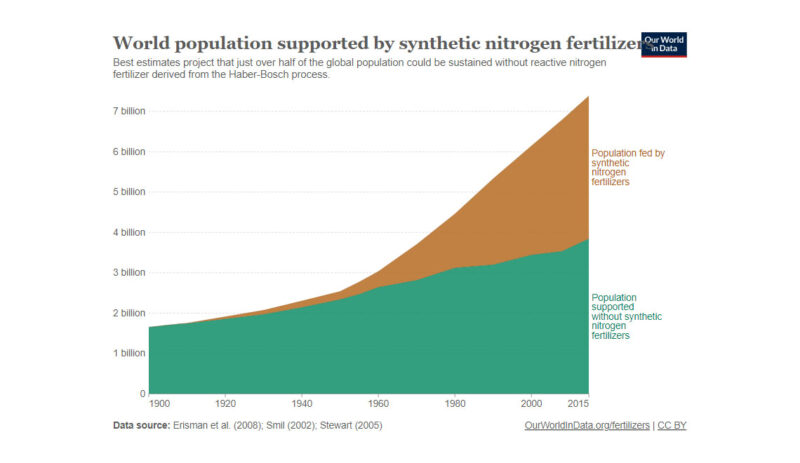
Currently, about 4% of the worldwide energy consumption is used to produce hydrogen. Around 90 million tons of hydrogen is produced in the world annually, as feedstock input to fertilizer production and for refining of oil. The fertilizers produced thanks to hydrogen allow to replenish the soil nutrients lost in agriculture. Without it, our food system could only sustain half of the current world’s population. If you need convincing, look at the following graph of the world’s population with and without fertilizers.
Regarding oil refining, the contribution of hydrogen in the desulfurization process allow to avoid the emission of sulfur oxides in the atmosphere during combustion, which makes our cities a lot healthier. If you want to see what happens without it, just look at the London Smog of 1952, caused by excessive sulfur oxide emissions and was directly responsible for the death of 4000 people.
So far, we have just been talking about the services hydrogen is currently providing to humanity.
In the coming decades, hydrogen is expected to provide the following additional services to humanity:
- Heavy mobility: Buses, trucks and non-electrified trains are expected to be decarbonized using hydrogen, as they need range and rapid refueling, which can hardly be provided by electricity.
- High-grade heat for industry: Industrial processes such as steel production, glass production, cement production, need temperatures over 1000°C, which are hardly achievable with electricity but can be easily reached thanks to the energy density of hydrogen.
- Chemical feedstock for industry: One example is the reduction of iron oxide to produce steel, that is responsible for 4-5% of the global greenhouse gas emissions. It could be decarbonized by direct reduction of iron (DRI) using hydrogen.
- Grid balancing and off grid power supply: Hydrogen can store electricity for extended periods of time and give it back to the grid through fuel cells when needed, thereby supporting the integration of intermittent renewables to the electric grid.
It is very important to note however that almost all the hydrogen currently produced is grey hydrogen, produced through steam methane reforming (SMR), and that emits 10kg carbon dioxide for every kg of hydrogen produced. That is why hydrogen production is currently responsible for 3% of the global greenhouse gas emissions. Another important aspect to consider is that over 99% of the hydrogen produced in the world is used on-site, meaning transport of hydrogen is almost inexistant currently.
Now, let’s address the second part of the introduction question, why is hydrogen so costly to produce?
Producing green hydrogen (through the electrolysis of water) costs around 60kWh/kg hydrogen in electricity. That is the equivalent of a week of electricity consumption for an average Dutch household, to produce one kg of hydrogen! Therefore, the electricity price is the main driver for the business case of hydrogen. And there is where the real problem lies. Taking the example of The Netherlands, the average wholesale electricity price in 2023 was 100€/MWh. At this price it already costs 6€ of electricity per kg hydrogen produced. At this point, we haven’t even accounted for the fixed operating costs, the capital costs, etc. Taking all these into account, the levelized cost of hydrogen cost will end up in the range of 8-10€/kg. The problem is that conventional grey hydrogen is generally priced around 1-2€/kg in the fertilizer and refinery markets. Looking at the new use-cases, the price tolerance is mostly below 3€/kg hydrogen.
One doesn’t need a PhD in economics to understand that the business case does not add up for green hydrogen at the moment. The only sector that could potentially absorb these price levels is the heavy mobility sector.
So how do we make green hydrogen cheaper?
As you may have deduced, the most significant parameter to affect is the renewable electricity price. So, we need to find cheap renewable sources of electricity to make green hydrogen cheaper.
And where can we find cheap renewables sources of electricity?
The key is to produce hydrogen in locations that meet the following criteria:
- The wind resources are excellent
- Large amount of space is available for renewable energy developments
- The local electricity demand is limited. This point is key to avoid the local market to drive the electricity prices up to levels that make green hydrogen production uneconomical
- Ideally also where solar is also competitive
There are many locations where these conditions are met, for example in Western Australia, Namibia, Southern Morocco, Northern Mauritania, Southern Chile, etc. The problem is that these locations are not the locations where a lot of hydrogen is needed. This means that, to drive mass adoption of green hydrogen, a significant amount of hydrogen transportation will be needed from the point of production to the point of use.
Remember we earlier wrote that hydrogen is almost never transported beyond the boundaries of the production plant. There are very good reasons for this. Hydrogen is the smallest molecule known to humans, meaning it diffuses very easily through storage vessels and valves. Hydrogen is also extremely flammable and extremely explosive. It has a minimum ignition energy of 0,02mJ, which is the smallest value among all molecules known to humans.

Ok, but with these difficulties, how are we expecting to transport hydrogen at scale in the future?
It turns out that there are a few technical solutions, which we will list and describe in the coming section:
Pressurized gaseous hydrogen:
This is the most straightforward way to store hydrogen, pressurize it up to 200-700bar and store it in a storage vessel. The advantage is that minimal infrastructure is needed, only a compressor and vessels are sufficient.
There are however many disadvantages to this technology. First, hydrogen is a very difficult gas to compress, due to its very low volumetric density. Therefore, special compressors are needed, that are very expensive and energy intensive. Hydrogen’s low volumetric density also means many storage vessels are needed to store significant amounts of hydrogen.
We mentioned earlier that hydrogen is a very small molecule, which means that special steel vessels and piping are needed to prevent hydrogen from escaping. There are other issues related to the specific thermodynamics of hydrogen, which we won’t detail here, but that compound further the difficulty of storing it in gaseous form.
Despite all these drawbacks, we do see a future for pressurized hydrogen storage in some use-cases. For instance, for onsite storage hydrogen storage for example, or in regions with hydrogen pipeline systems, pressurized hydrogen buffering is often a good solution. At Proton Ventures, we routinely include pressurized gaseous hydrogen storages in our green ammonia production facilities to buffer between the electrolyser and the ammonia loop.
Liquified hydrogen:
To increase the volumetric density of hydrogen, a solution is to liquify the hydrogen and transport it as a liquid. Here, we would mimic the way methane is transported in the form of LNG. Unfortunately, that’s where the comparison ends, because methane is liquid at -162°C whereas hydrogen is only liquid at -252°C. Reaching such a low temperature in a refrigeration system is incredibly difficult. With Best Available Technology, the current energy cost of liquefaction is 15-20kWh/kg. That’s about 30% of the energy used for hydrogen production, that needs to be added to liquify it. That is just the beginning of the challenge, next hydrogen needs to be transported and stored at import location at -252°C, meaning very high boil-off rates, that either must be reliquefied, or vented. The first option requires an advanced on-board refrigeration system. The second option means loss of product and release of a potent greenhouse gas (hydrogen has a global warming potential of 11), defying the original purpose of decarbonization. A lot of research is currently underway on refrigeration systems and vessels for liquid hydrogen storage, meaning improvements in its storage and handling are expected. Thermodynamic simulations even seem to suggest that large-scale transportation of liquified hydrogen would be economically viable in the long-term. However, the technical and infrastructure barriers are too high in the short and medium term to make it a viable large-scale hydrogen carrier.
Liquid Organic Hydrogen Carriers (LOHCs):
These are a family of carbon-based molecules, such as toluene or dibenzyltoluene, that can easily bind and release hydrogen on-demand. The big advantage of LOHCs is that they can carry hydrogen at large scale at atmospheric temperature and pressure. LOHCs can also use existing infrastructures for oil and LPG transportation, meaning the infrastructure challenges are minimal.
On the downside, LOHCs need a hydrogenation and dehydrogenation facility at the export and import locations to bind and release the hydrogen from their organic carriers. These facilities will have to be purpose-built. On the import side, another challenge is that dehydrogenation reactions are endothermic reactions, meaning heat needs to be added to allow the dehydrogenation process to occur. The problem is that hydrogen is being imported to meet energy demands, but energy needs to be added first to release it, which defies the purpose. Other carriers like ammonia and methanol, that we will see later, have the same issue, showing that the chemical thermodynamics of hydrogen are rigged against its transportation and conversion.
There are two other significant drawbacks of LOHCs, namely transportation costs and toxicity. Let’s start with toxicity. Toluene is a molecule known for being a strong irritant to the body tissues and for its carcinogenic effects. That is not a showstopper, and it is also less dangerous in the short-term than ammonia, which we will see later, but means the standards and procedures for storage and handling must stillbe very solid. Regarding transportation costs, LOHCs have the problem that they have a very low hydrogen content per mass of loaded carrier, about 6%, meaning that only 6% of the mass transported is effectively hydrogen, and 94% is the organic carrier. The second problem is that when unloaded at the import location, the carrier must be shipped back to the export location with 94% of its mass, which entails higher total transportation costs versus alternative hydrogen carriers.
There are a few concrete projects currently involving LOHCs, for instance Vopak and Hydrogenious are currently building 2 industrial LOHC dehydrogenation facilities in Dormagen and Rotterdam, showing there is potential for LOHCs to contribute to hydrogen transportation to Europe.
Methanol: Methanol is a molecule with the chemical formula CH3OH and a commonly produced and traded commodity in the world. Around 90 million tons of methanol are produced in the world annually. Methanol is a building block to many commonly used plastics, fine chemicals and transportation fuels. In the short and medium term, methanol is foreseen to replace fuel oils as a bunker fuel for the maritime sector. Methanol has the big advantage of being a liquid at ambient pressures and temperature, meaning the transportation challenges are minimal, and the infrastructure challenges as well. Methanol synthesis, storage and transportation are therefore mature and standardized processes. Methanol cracking suffers the same problem of endothermicity at import location, but this can be solved more easily than LOHCs by burning part of the methanol to compensate the heat losses. Seen from that perspective, methanol looks like the perfect hydrogen carrier…. But methanol has one major Achilles heel, which will weigh very heavily on every future hydrogen carrier decision, and that is its carbon atom.
To produce green methanol, one molecule of carbon dioxide and two molecules of hydrogen are required. The question is: Where does this molecule of carbon dioxide come from? There are roughly 3 options to choose from: An industrial waste stream, a biogenic carbon or direct air capture.
- Industrial waste streams could be an option in the short term, but most industrial processes will be decarbonized in the coming decades, meaning that industrial processes having a pure carbon dioxide waste stream will become scarce in the future. On top of that, as dehydrogenation emits carbon dioxide back to the atmosphere, using industrial waste streams for methanol is not truly decarbonization.
- Biogenic carbon sources will have a lot of competition in the future from sustainable aviation fuels and other hard-to-abate sectors, making it also a scarce and expensive resource in the future.
- Direct air capture (DAC) which is the absorption of carbon dioxide directly from the atmosphere, is a very energy intensive and expensive process. Recent studies place the cost of DAC currently at 600-1000$/T, with a potential to reach 230-540$/T by 2050 (ETH Zurich). That shows that using DAC for methanol production does and will weigh heavily on the economics of the methanol value-chain.
Because of these limitations, green methanol is likely to only play a significant role in the decarbonization of the production of chemical products and probably also of heavy transportation. The supply issues for its carbon content will most likely prevent it from being used in use-cases requiring hydrogen.

Ammonia: It is the second most widely produced chemical in the world with 190 million tons of global production annually, mostly for the fertilizer sector. Like methanol, ammonia has been widely stored, handled and transported for over a century, making the storage and handling procedures very mature. There are over 180 ammonia terminals, and LPG infrastructures can be converted to ammonia handling with minor upgrades.
Ammonia has a higher mass hydrogen density, at 17,5%, than LOHCs (6%) and methanol (12,5%). It even has a 30% higher volumetric hydrogen density than liquid hydrogen itself. That allows to reduce the transportation costs, as space and weights can be optimized in ships.
Versus methanol, ammonia has the big advantage of having a nitrogen base and not a carbon base and nitrogen can be very easily drawn from the atmosphere and released back without environmental impact or technical complications.
Versus LOHCs, ammonia has the advantage of having a higher hydrogen density and it does not need to be shipped back to import location. As with methanol and LOHCs, the cracking of ammonia is an endothermal reaction, meaning energy input is required at import location. However, as with methanol, that input can be supplied by the combustion of around 20% of the ammonia content.
Ammonia is, however, not as easy as methanol or LOHC to store and handle, as it is a very toxic substance, being lethal at an exposure of 15 minutes at a few hundred ppm. On top of that, ammonia is a gas at atmospheric temperature and pressure, meaning it needs to be kept in liquid state by either refrigerating to -33°C, or by pressurizing it to 8bar. These conditions are a lot easier to maintain than keeping hydrogen liquid, but it is not as easy as chemicals that can be stored at atmospheric conditions.
Looking at current market developments, ammonia is clearly leading the green molecule infrastructure developments for intercontinental hydrogen carrier transportation. Over 250 green ammonia production plants and 30 ammonia terminals are currently under development in the world, showing that project developers are seeing the advantageous cost-benefit ratio of ammonia in the future green hydrogen economy.
Other hydrogen carriers: In this article, we have looked at the main hydrogen carriers, but there are many other alternatives, such as formic acid, iron-oxide, glass adsorption, etc. These have a low commercial maturity for now and their maturation is uncertain, meaning we won’t detail them here. Also important to note that green steel has been excluded from the analysis as it cannot readily by reconverted into hydrogen.
Now, comes the answer to the question you have certainly waited for since starting this article, which is the best hydrogen carrier?
And the answer to this is… It depends…
As we have seen, every hydrogen carrier entails technical and economic challenges, currently preventing their wide-scale adoption. But as cheap green hydrogen is needed to decarbonize our economies, these challenges will have to be overcome, and companies across the world, including Proton Ventures, are working very hard every day to overcome these challenges.
In general, a few rules of thumb for the selection of hydrogen carriers are:
- If the final use of the hydrogen is in the form of ammonia or methanol for shipping or chemical use, then the carrier selection is straightforward.
- If hydrogen is available in close vicinity of the point of use, then pressurized gaseous hydrogen is usually the way to go.
- If your final use-case is hydrogen and the hydrogen needs to be shipped intercontinentally, ammonia is most often the preferred carrier, but LOHCs, methanol and liquid hydrogen should also be looked at on a case-by-case basis.
At the end, there is no one-size fits all in the energy transition, and a mix of hydrogen carriers will be needed to unlock the potential of green hydrogen. With the right choices of technological solutions, hydrogen could contribute to decarbonize 15-20% of the global greenhouse gas emissions.
Proton Ventures works daily with its clients to unlock the potential of green hydrogen. We support our clients with their technological choices for hydrogen transportation and with the design of production and storage facilities for green molecules. We can support our clients along the entire project development value-chain, from pre-feasibility assessments to owner’s engineering services during the construction phase.
If you have a project and need a recognized technical expert to bring it to the next phase, don’t hesitate to reach out to Proton Ventures!